
- 997556699
- [email protected]
Pharma Consulting
02
Oct
Oct

IMDRF management comittee announces DIGEMID (Peru) as new affiliate member
by Pharma Consulting | IMDRF, medical devices | 0 Comment
The General Directorate of Medicines, Supplies and Drugs (DIGEMID) of Peru was announced as new affiliate member at the 26th International Medical Device Regulators Forum (IMDRF) Management Comittee Session held in September, 2024 and hosted by the United States of America. What's the IMDRF? The International Medical Device Regulators Forum or IMDRF is a group
05
Mar
Mar

Steps to enter the Peruvian market (2024 Update)
by Pharma Consulting | market access | 0 Comment
Steps to enter the Medical Device, Dietary Supplements, and Cosmetics Market in Peru Perú has over 33 million residents, and is a strong, developing market for foreign manufacturers. Our local industry does not satisfy our population demands and we depend heavily on import products mainly from China, US, India, Israel, Brazil, Colombia, among other industrialized
08
Jan
Jan

Food Supplements Registration and Regulations in Peru
by Pharma Consulting | dietary supplements | 0 Comment
In Peru, the General Directorate of Medicines, Supplies, and Drugs (DIGEMID) is the National Authority that regulates the food suplements market. What a food supplement is (and what it is not) in Peru A food supplement is any product that contains vitamins, minerals, amino acids, vegetal extracts, enzymes, chemical substances such as glucosamine, methylsulfonylmethane, hyaluronic
07
Aug
Aug

Extended Foreign GMP Acceptance
Sponsors of product registrations and importers will benefit from this regulation since it will enable the uninterrupted flow of pharmaceutical products from and to Peru until 2028. Good Manufacturing Practices (GMP) certificates for pharmaceutical products (including medicines, biologics, and dietary supplements, among others) issued by foreign countries' national authorities will be accepted as valid by
06
Nov
Nov

Workout dietary supplements may contain steroids U.S. FDA warns
by Pharma Consulting | dietary supplements | 0 Comment
The U.S. Food and Drug Administration (FDA) has found that many bodybuilding (workout) products may contain anabolic steroids or steroid-like substances in their composition. Such ingredients included illegally or not declared by manufacturers may pose serious health risks to consumers, for example, severe liver (hepatic) injury, kidney damage, heart attack, stroke, pulmonary embolism, deep vein
01
Oct
Oct
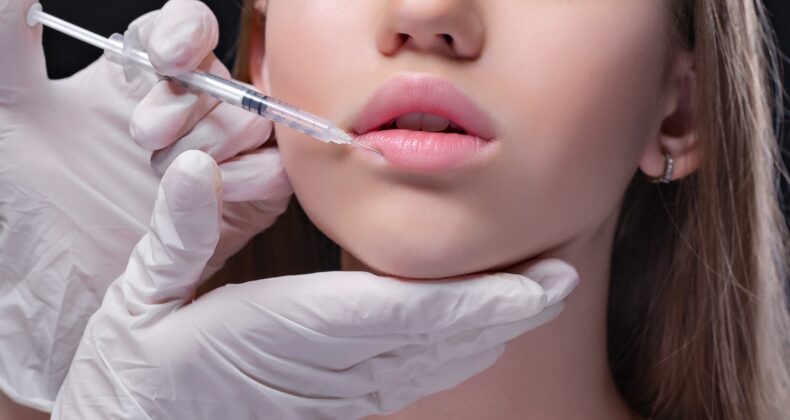
Dermal fillers as medical devices
by Pharma Consulting | medical devices | 0 Comment
Dermal fillers, injectable implants, or soft tissue fillers are gelatin-like products intended to be injected under the outer layers of the skin to improve the appearance of wrinkles, depressions, and defects such as scarred tissue, or correct loss of volume on different body parts, allowing a smoother and fuller appearance. These products are considered medical
28
Apr
Apr

Characteristics of the regulation of medical devices and equipment in Peru
by Pharma Consulting | biomedical equipment, medical devices | 0 Comment
Regulatory Authority In Peru, the General Directorate of Medicines, Supplies, and Drugs (DIGEMID) is the National Authority that oversees the medical devices, equipment, pharmaceutical products, personal care, and health products market. Summary of Peruvian regulatory legislation Is it necessary to obtain authorization to operate as a company prior to marketing restricted products? Yes, according to
26
Apr
Apr

Regulatory aspects of dietary products and sweeteners in Peru
by Pharma Consulting | dietary supplements, registration | 0 Comment
Regulatory aspects of dietary products and sweeteners The General Directorate of Medicines, Supplies, and Drugs (DIGEMID), during the first "Virtual technical meeting on the registration process of nutritional supplements and sweeteners", established some criteria to be taken into account by users interested in registering these products. Requirements for the health registration of dietary supplements (nutritional)
10
Apr
Apr

Dietary supplements must not claim to cure, mitigate, or prevent chronic diseases
by Pharma Consulting | dietary supplements | 0 Comment
The FDA warned 10 companies for illegally selling dietary supplements that claim to cure, treat, mitigate, or prevent chronic diseases, like diabetes mellitus. The Administration urges consumers not to consume products that have not been evaluated by the FDA to be safe or effective for their intended use. In Perú, the General Directorate of Medicines,
04
Jan
Jan
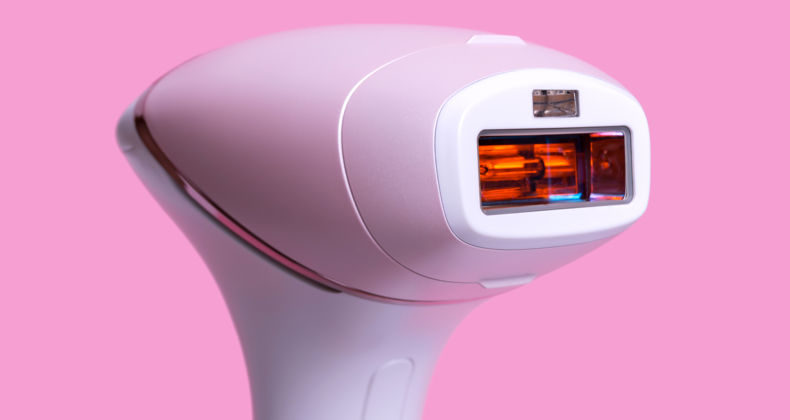
Registration of laser, IPL and radiofrequency equipment for use in aesthetics in Peru
by Pharma Consulting | dispositivos médicos, medical devices, registration, registro sanitario | 0 Comment
Laser, IPL and radiofrequency equipment for use in aesthetics A device for use in aesthetic medicine is classified biomedical, active, non-invasive, or minimally invasive device that acts through the administration or exchange of energy in the form of non-ionizing radiation, to or from the human body. Most of the equipment in this category belongs to